What is Our Product
Our product is a prosthetic heart valve that is implantable at all four valve positions: the aortic, mitral, pulmonary, and tricuspid. Our valve prosthetic is capable of expanding. The exapanbilty allows for the valve ring to increase in size with the patient over time,thus decreasing prosthesis-patient mismatch. Our valve is composed of cutting edge nitinol material that is used for our stent and hand selected bovine pericardium tissue that ensures the best match for every device.
The utilization of nitinol for the stent allows us to manipulate the size of the heart valve prosthesis due to the shape-memory and superelasticity properties of the material. Two pieces of medical cloth were incorporated in the design of the prosthesis to protect the tissue leaflets from direct contact and friction with the nitinol stent.

What Makes Us Different
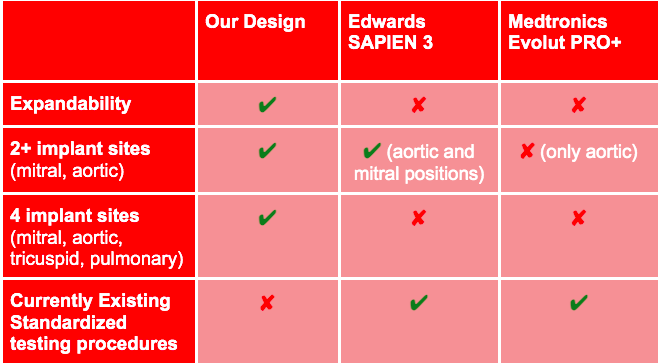
Unlike our competitors, our heart valve prosthesis features expandability to account for patient growth and aims to be implanatable at the mitral, aortic, tricuspid, and pulmonary positions. While there are no current standardized testing procedures for this unique feature of a heart valve prosthesis, our device allows for verification testing of the heart valve leaflet structure at multiple positions, paving the regulatory pathway for future iterations of a prosthesis with an expandable base. Along with a working heart valve prosthesis, the outcome of our design and testing process would be a standardized testing procedure for this newly imagined type of prosthesis.
Market Strategy
Compared to current devices on the market, our product possesses advantages that many others do not:
1. Our heart valve prosthesis can be installed through both transcatheter and open heart procedures
2. Our design is expandable, which allows the device to account for patient growth and ViV reinterventions
3. Our heart valve prosthesis can be implanted at multiple positions in the heart
As a small start-up medical device company, when it comes to selling our devices, the most important goal is to amass recognition. To do so, our company will be participating in medical device exhibitions and conferences to showcase our products to medical professionals. Due to the unique advantages of our product, which effectively targets existing problems in the artificial heart valve industry, we believe that our product will attract enough attention from people in the field, especially medical device distribution enterprises.
Our team will be cooperating with doctors to provide complimentary training for medical practitioners on how to implant our device through open heart surgery and transcatheter techniques. This will allow more doctors to master the procedures related to implanting our device and increase the efficacy of our product.
Our team at the Fall 21 BioENGINE poster presentation